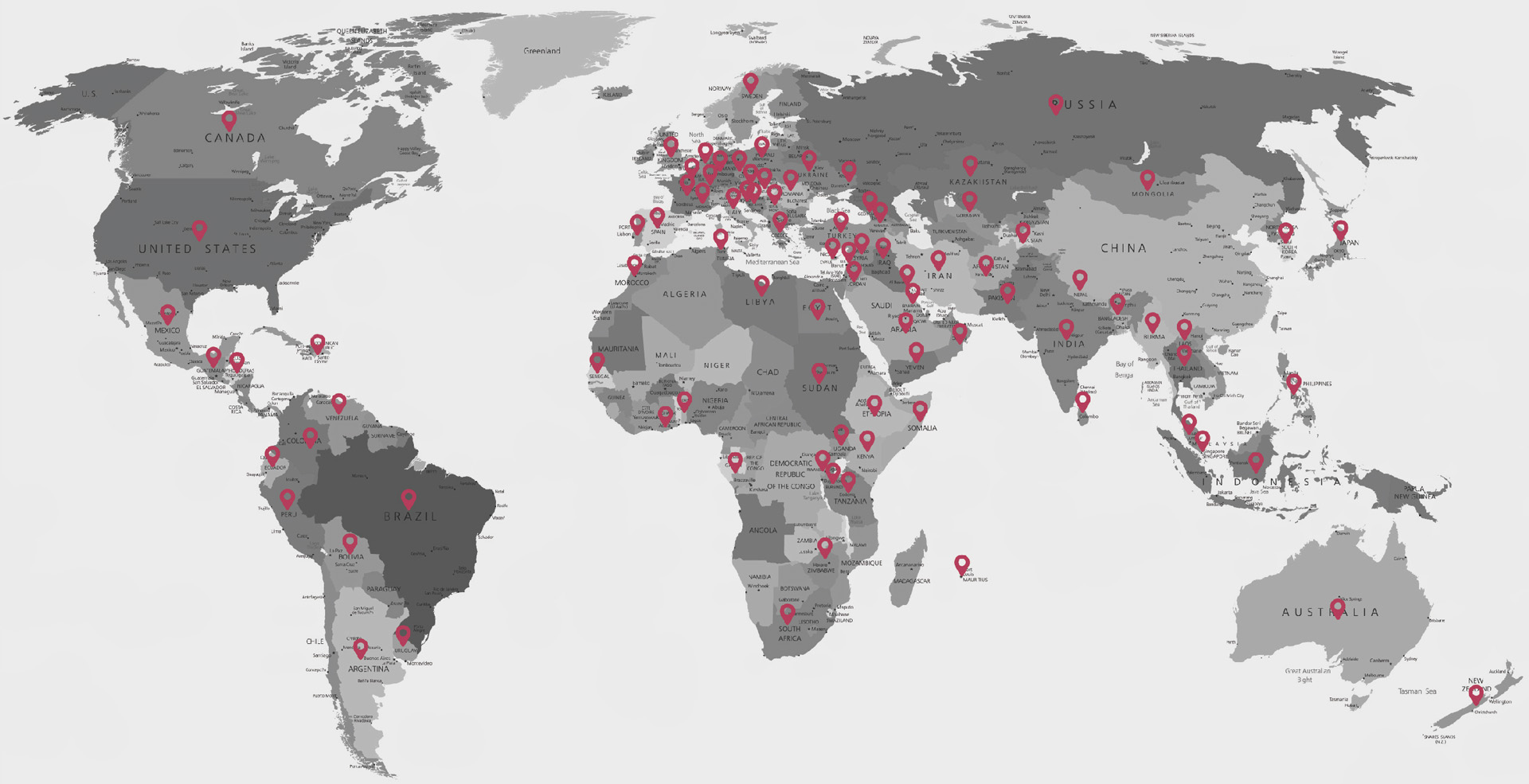
Yonker (Xuzhou Yongkang Electronic Science Technology Co., Ltd.) e hiwere na 2005, anyị bụkwa ụlọ ọrụ mmepụta akụrụngwa ahụike ọkachamara a ma ama n'ụwa niile nke na-ejikọta nyocha na mmepe, mmepụta, ire ahịa na ọrụ. Ugbu a, Yonker nwere ụlọ ọrụ enyemaka asaa. Ngwaahịa ndị dị na ngalaba atọ na-ekpuchi ihe karịrị usoro 20 gụnyere oximeters, ihe nlele ndị ọrịa, ECG, pump sirinji, ihe nlele ọbara mgbali elu, ihe nchekwa oxygen, nebulizers wdg, nke a na-ebuga na mba na mpaghara karịrị 140.
R&D na Mmepụta
Yonker nwere ebe nyocha na mmepe abụọ na Shenzhen na Xuzhou, ya na ndị otu nyocha na mmepe nke ihe dị ka mmadụ 100. Ugbu a, anyị nwere ihe fọrọ nke nta ka ọ bụrụ patent 200 na akara azụmaahịa enyere ikike. Yonker nwekwara ntọala mmepụta atọ na-ekpuchi mpaghara nke mita 40000 nke nwere ụlọ nyocha onwe ha, ebe nnwale, ahịrị mmepụta SMT nwere ọgụgụ isi, ebe a na-arụ ọrụ na-enweghị uzuzu, nhazi ebu ziri ezi na ụlọ ọrụ ịkpụzi ihe, na-emepụta usoro mmepụta na njikwa mma zuru oke na nke a na-achịkwa ọnụ ahịa. Ihe mmepụta ya fọrọ nke nta ka ọ bụrụ nkeji 12 iji gboo mkpa ahaziri nke ndị ahịa ụwa.
Ndị otu ọrụ mgbe ahịa gasịrị
N'okpuru nduzi nke ụkpụrụ nke "eziokwu, ịhụnanya, arụmọrụ, na ibu ọrụ", Yonker nwere sistemụ ọrụ onwe ya mgbe eresịrị ahịa maka nkesa, OEM na ndị ahịa ikpeazụ. Ndị otu ọrụ ntanetị na nke na-anọghị n'ịntanetị bụ ndị na-ahụ maka usoro ndụ ngwaahịa niile. Iji melite arụmọrụ ọrụ, ndị otu ahịa na ọrụ Yoner na mba na mpaghara 96, n'ime awa ise iji zaa usoro njikọ arịrịọ, inye ndị ahịa nkwado teknụzụ ọkachamara karịa.
Njikwa na Asambodo Ogo
Usoro nlekota ogo ọrụ nke Yonker na-enyere aka karịa nhazi ngwaahịa Yonker zuru ụwa ọnụ. Ruo ugbu a, ihe karịrị ngwaahịa 100 nwere CE, FDA, CFDA, ANVISN, TUV ISO13485, CMD ISO9001 na asambodo ndị ọzọ. Nnyocha ngwaahịa na-ekpuchi IQC, IPQC, OQC, FQC, MES, QCC na usoro njikwa ọkọlọtọ ndị ọzọ, a họpụtara Yonker dị ka National High-tech Enterprise, National Intellectual Property Advantage Enterprise, Jiangsu Medical Device Manufacturing Enterprise Member Unit. Yonker anọgidewokwa na-enwe mmekọrịta mmekọrịta ogologo oge na Renhe Hospital, Respironics, Philips, Suntech Medical, Nellcor, Masimo na ụdị ndị ọzọ a ma ama.
Ọhụụ ụlọ ọrụ
Na-achọ ihe ga-eme ka ndụ na ahụike dịrị gị mma
Ngwaọrụ ahụike 100 kachasị elu na China nke afọ 2025
Ụkpụrụ isi ụlọ ọrụ:Ezi obi, ịhụnanya, arụmọrụ na ibu ọrụ
Ọrụ ụlọ ọrụ:Na-agbasosi ike mgbe niile inye ndị ahịa ngwaahịa dị mma na ọnụ ahịa dị elu ma na-akpali obi ndị mmadụ
Ngalaba enyemaka nke Yonker Group, Periodmed, ekpughere ngwaahịa ahụike ọhụrụ na 2024 Shanghai CMEF












Ụlọ ọrụ enyemaka ahụike nke Yonker Group, Periodmed Medical, ga-amalite ọrụ mbụ ha na Dubai Arab Health Exhibition nke afọ 2024.












Ihe ngosi Ụlọ Ọgwụ Mba Nile nke Düsseldorf na Ngwa Ahụike na Germany








2023 China (Shenzhen) 88th China International Medical Equipment (Mgbụsị akwụkwọ) Expo
-2.png)
-15.jpg)
-71.jpg)
-2.jpg)
-37.jpg)
-14.jpg)
-24.jpg)
-13.jpg)
-35.jpg)
-33.jpg)
-29.jpg)
-21.jpg)
-32.jpg)
-16.jpg)
-6.jpg)
-39.jpg)
Ụlọ ngosi ahụike Yonker na Jakarta, Indonesia na Hall B 238 na 239








Ngwaahịa Yonkermed nke a gosipụtara na ihe ngosi ahụike South Africa nke afọ 2023




Ihe ngosi ngwaọrụ ahụike ọhụrụ nke 2023 Yonker








Ndị otu ọkachamara








Nkwanye ugwu azụmaahịa azụmaahịa
A họpụtara Yonker dị ka National High-tech Enterprise, National Intellectual Property Advantage Enterprise, Jiangsu Medical Device Manufacturing Enterprise. Yonker anọgidewokwa na-enwe mmekọrịta mmekọrịta ogologo oge na Renhe Hospital, Respironics, Philips, Suntech Medical, Nellcor, Masimo na ụdị ndị ọzọ a ma ama.
Ruo ugbu a, ihe karịrị ngwaahịa 100 nwere CE, FDA, CFDA, ANVISN, TUV ISO13485, CMD ISO9001 na asambodo ndị ọzọ. Nnyocha ngwaahịa na-ekpuchi IQC, IPQC, OQC, FQC, MES, QCC na usoro njikwa ọkọlọtọ ndị ọzọ.







Respironics Etco2

Ngalaba ọkụ Philips

Onye na-eweta modulu ọbara mgbali elu zuru ụwa ọnụ

45% nke ahịa nke SPO2 zuru ụwa ọnụ


